Abstract

Background Recent data show that RT on PET-negative sites after chemo-immunotherapy in early stage DLBCL can be avoided. Nevertheless, bulky sites at diagnosis are still irradiated after rituximab-chemotherapy (R-CT), whereas residual uptake area (RUA at other sites are considered as failure. As PET negativity is mandatory to define complete remission (CR), we hypothesized that PET-neg areas after R-CT do not need consolidation RT independently from their size at onset
Aims: To assess the role of RT in PET-neg and in PET-pos low-risk DLBCL patients after R-CT
Methods: The DLCL10 protocol was a phase II study of patients (pts) >=18 years with low risk DLBCL according to the MiNT trial, (aa IPI 0 and bulky, aa IPI 1 +/- bulky) conducted in 19 FIL centers. Pts were treated with 6 courses of RCHOP and final response was evaluated with FDG-PET. Both pre and post treatment PET scans were centrally reviewed through the Widen web platform by a panel of 5 nuclear medicine experts. Positive scans were those centrally classified with Deauville score 3-4-5 by the first 2 concordant reviewers. Pts with one RUA received RT, 36 Gy involved-site, regardless of bulky disease at onset, while those with multiple RUA were shifted to salvage systemic therapy. Primary aim was to obtain a 2-year PFS of at least 85% for post R-CT PET-neg pts. Secondary endpoints were OS and response.
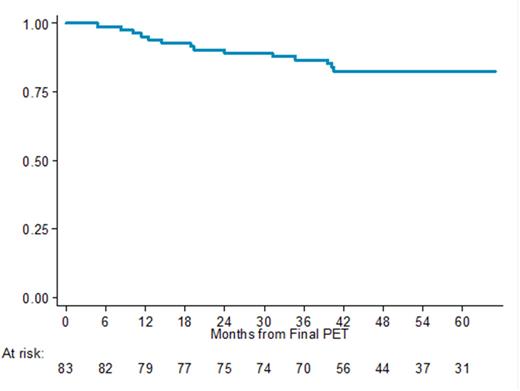
Results: From January 2012 to December 2017, 115 consecutive pts were screened, and 110 were evaluable. Median age 58 years (47-65); M:F 61 /49; DLBCL de novo 90%, aa IPI 0 16 , aa IPI 1 94 (20% with bulky mass), bulky disease in the whole series 35 pts ; RCHOP-14 /RCHOP-21 73/37. At the time of the present analysis median follow-up was 63 months and 13 pts died (3 lymphoma, 2 acute toxicitiy, 2 late toxicity, 2 secondary neoplasm, 2 complication from allo-transplant, 1 other causes, 1 unknown). A total of 105 pts completed the R-CT program, while five were discontinuated for lymphoma progression (1), toxicity (2, both died), histological review (1) and patient dispersion (1). At end of treatment, 83 patients had PET-neg, whereas 17 had single RUA and received involved -site RT. In PET-neg patients, PFS was 90.6% (95% CI 81.1-95.4) at 2 years and 82.48% (95% CI 78.4-94.3) at 5 years. OS was 96.37% (95% CI 89.17-98.81) at 2 years and 87.81% (95% CI 77.62-93.54) at 5 years. After RT, 15 pts reached CR, one PR and one was not evaluable. None of them relapsed. Thus, all patients with positive focal RUA after R-CT were cured with involved-site RT. Concerning the 35 pts with bulky disease, 21 reached PET-neg and 14 had single RUA after R-CT and were thus irradiated (1 PD). There were two relapses in the PET-neg/not irradiated group, but only one in previously bulky site. In the PET-pos /RT group no relapse occurred. In the total population, 5 -year PFS and OS are 80.9% (95%, CI 79.28-92.18) and 87.1% (95%, CI 78.66- 92.38), respectively.
Conclusion: Our data suggest that irradiating only sites of unique PET RUA, regardless of bulky at onset, can be considered as a reasonable strategy for low risk DLBCL pts. In cases with bulky disease, PET-driven RT allowed RT sparing in approximately half of patients in this small series. Moreover, consolidation RT in those with focal residual PET positivity, guaranteed excellent prognosis (17/17 cured) and can be considered as a valid option
Disclosures
Balzarotti:Servier: Membership on an entity's Board of Directors or advisory committees; Gentili: Membership on an entity's Board of Directors or advisory committees; Astrazeneca: Honoraria; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria; Takeda: Honoraria. Tucci:Janssen: Membership on an entity's Board of Directors or advisory committees; MSD: Honoraria; Sanofi: Membership on an entity's Board of Directors or advisory committees; Gentili: Membership on an entity's Board of Directors or advisory committees; Kiowa Kyrin: Honoraria; Takeda: Honoraria. Cavallo:Amgen: Other: Expenses for EHA virtual meeting; Roche: Membership on an entity's Board of Directors or advisory committees, Other: Expenses for Ash meeting; Takeda: Other: Expenses for ICML virtual meeting; Servier: Speakers Bureau. Zilioli:Gentili: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Other: travel expenses, Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy; MSD: Membership on an entity's Board of Directors or advisory committees; Janssen: Other: travel expenses, Speakers Bureau. Vitolo:AbbVie, Incyte, Janssen, Gilead Sciences: Speakers Bureau; Seagen, Genmab, Incyte, Costellation, Bayer, Regeneron: Consultancy. Santoro:AstraZeneca: Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Eli-Lilly: Speakers Bureau; Sandoz: Speakers Bureau; Eisai: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Speakers Bureau; Bayer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Merck Sharp & Dohme: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Speakers Bureau; Abb-vie: Speakers Bureau; Amgen: Speakers Bureau; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Speakers Bureau; Incyte: Consultancy; Sanofi: Consultancy. Gaidano:Astra-Zeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal